The discovery of Alzheimer’s has spurred vast research into the underlying mechanisms of this devastating neurodegenerative disease. Renowned neuroscientist Beth Stevens has shifted our understanding of microglial cells, which act as the brain’s immune system, thereby unveiling their critical role in Alzheimer’s treatment. These cells monitor and maintain brain health by clearing out damaged neurons; however, their malfunction can lead to aberrant synapse pruning, which is linked to the progression of Alzheimer’s. Stevens’ groundbreaking work at Boston Children’s Hospital is paving the way for innovative strategies to combat the disease, aiming to enhance the lives of approximately 7 million Americans affected. As we continue to navigate the complexities of brain health and immune responses, the significance of this research in Alzheimer’s discovery cannot be understated.
The exploration into the origins of Alzheimer’s has led to significant breakthroughs in our grasp of neurodegenerative disorders. Researchers like Beth Stevens are revolutionizing the study of brain immunity by focusing on microglial cells, the brain’s primary defenders against cellular damage. These findings are not only shedding light on the pathology of Alzheimer’s disease but are also opening doors to new therapeutic avenues. The intricate relationship between brain immune response and neuronal health has become a focal point of modern neuroscience, emphasizing the need for ongoing research in this critical area. As awareness of Alzheimer’s grows, the impact of such research reiterates the essential link between foundational science and potential therapeutic innovations.
Understanding Microglial Cells in Alzheimer’s Disease
Microglial cells are often described as the brain’s immune system, playing a critical role in maintaining neuronal health and homeostasis. These specialized cells patrol the brain and spinal cord, actively seeking out signs of damage or infection. In the context of Alzheimer’s disease, their function becomes especially crucial, as microglia engage in processes of clearing away dead or damaged neurons and protecting the brain from neurodegenerative threats. However, Beth Stevens, a leading neuroscientist, has highlighted that in conditions like Alzheimer’s, microglial activity can sometimes go awry, leading to excessive pruning of synapses that are vital for communication between neurons. This aberrant pruning is a significant contributing factor to the progression of Alzheimer’s disease and other neurodegenerative disorders, which challenges traditional views of microglia as solely protective agents in the brain.
The implications of Stevens’ research are vast, showcasing that a detailed understanding of microglial behavior could pave the way for innovative Alzheimer’s treatments. By focusing on the mechanisms of microglial activity, researchers can identify specific pathways that could be targeted to enhance their protective functions or mitigate their harmful effects. This insight is instrumental in creating biomarkers for early detection of Alzheimer’s, potentially allowing for interventions before irreversible neuronal damage occurs. As the number of Alzheimer’s cases continues to surge, understanding the role of microglial cells becomes a cornerstone of developing effective therapies and improving the quality of life for millions of individuals affected by neurodegenerative diseases.
The Transformative Research of Beth Stevens
Beth Stevens’ groundbreaking research on microglia has significantly transformed our understanding of Alzheimer’s disease. Her work has revealed that these cells, while integral to brain health, can become detrimental when their functions are dysregulated. The core of Stevens’ research lies in her exploration of how microglia prune neural connections during brain development and how this process can lead to neurodegeneration later in life. Such insights underline the complexity of microglial behavior and its potential impact on Alzheimer’s treatment methodologies. By emphasizing the scientific curiosity that guided her explorations, Stevens highlights the importance of basic research in uncovering profound connections between immune responses in the brain and neurodegenerative diseases.
Stevens’ findings have far-reaching implications not only for Alzheimer’s disease but also for other neurodegenerative conditions like Huntington’s disease. Her laboratory’s exploration into microglial cells has laid the groundwork for identifying potential biomarkers that could allow earlier diagnosis and intervention strategies. This commitment to curiosity-driven science showcases how fundamental research can lead to unexpected medical advancements. As Stevens aptly puts it, the path from initial scientific inquiry to groundbreaking discoveries in Alzheimer’s treatments exemplifies the essential role of basic science in medical progress. This pioneering work not only enriches our understanding of the brain’s immune system but also fosters hope in the fight against debilitating neurodegenerative diseases.
The Role of the Brain Immune System in Alzheimer’s Treatment
The brain immune system, mainly composed of microglial cells, plays a pivotal role in the maintenance of neuronal integrity and overall brain health. In circumstances where the immune response is disrupted, as seen in Alzheimer’s disease, neurodegeneration can accelerate. Stevens’ research emphasizes that proper microglial function is critical in clearing amyloid plaques and other pathological markers associated with Alzheimer’s. Understanding the dual nature of microglia—as both protectors and potential aggressors—is vital in developing therapeutic strategies. By harnessing their innate capabilities, scientists can design treatments that either enhance their beneficial properties or inhibit their harmful effects, thereby revolutionizing Alzheimer’s treatment protocols.
Targeting the brain immune system presents a novel approach in combating Alzheimer’s disease. Recent advances suggest that therapies aimed at modulating microglial activity could yield significant benefits in halting or even reversing disease progression. For instance, therapeutic agents that can enhance the synaptic pruning abilities of microglia may clear out harmful amyloid deposits, leading to improved cognitive function in affected individuals. This intersection of immunology and neurology represents a turning point in the therapeutic landscape for Alzheimer’s, as it shifts the focus from mere symptomatic relief to addressing the underlying mechanisms of the disease. Furthermore, ongoing research inspired by Stevens’ findings continues to uncover additional roles of microglial cells in brain health, promising to inform more effective treatment strategies.
Emerging Biomarkers for Alzheimer’s Disease Detection
Beth Stevens’ pioneering work on microglial cells has opened up expansive avenues for identifying early-stage biomarkers for Alzheimer’s disease. Biomarkers are biological indicators that can signal the presence or progression of a disease, and in the context of Alzheimer’s, they hold immense potential for transforming how the disease is diagnosed and managed. By examining the behavior of microglia and their interactions with neuronal cells, researchers can potentially identify specific molecular changes that precede the onset of cognitive decline. This early detection is crucial, as it allows for intervention strategies to be implemented at a stage when they may be most effective.
Furthermore, advancements in biomarker research could lead to the development of diagnostic tools that not only help identify Alzheimer’s but also monitor its progression. Utilizing the characteristics of microglial activity as a framework for these biomarkers could result in innovative blood tests or imaging techniques capable of providing real-time insights into brain health. The ongoing work in Stevens’ lab underscores the importance of understanding the brain’s immune system as a source for these new biomarkers. As such, the integration of immunological and neurological research will be key in creating a robust framework for diagnosing Alzheimer’s disease more accurately and earlier than ever before.
The Future of Alzheimer’s Research and Treatment
The future of Alzheimer’s research is poised for significant advancements, largely due to foundational studies like those conducted by Beth Stevens. As researchers continue to unravel the complexities of microglial function, we may see revolutionary shifts in how we approach Alzheimer’s treatment. With the aging population in mind, the urgency to find effective therapies has never been greater. The burgeoning understanding of the brain’s immune system and its role in neurodegeneration promises to transform not just how we treat Alzheimer’s, but potentially, how we can prevent its onset.
Innovative treatments that arise from this research could include targeted therapies that modulate microglial activity, thereby improving synaptic functioning and reducing neurotoxic effects. Moreover, as diagnostic capabilities improve through the identification of novel biomarkers, earlier and more precise interventions may become a reality, drastically improving patient outcomes. The insight gained from Stevens’ research stimulates hope and potential avenues for preventing Alzheimer’s, setting a promising trajectory for future inquiries into neurodegenerative diseases.
Impacts of Federal Funding on Alzheimer’s Research
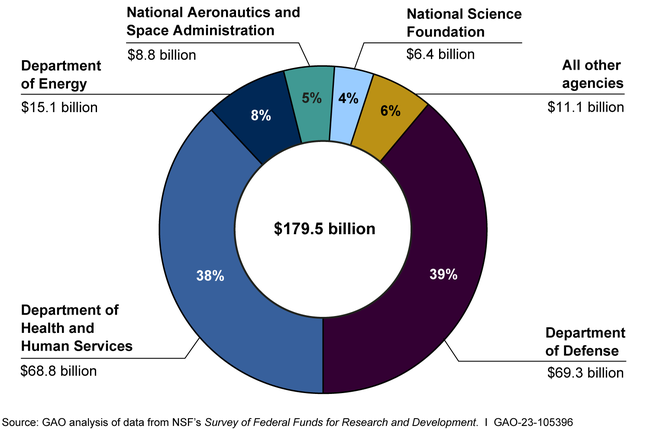
Federal funding has played a crucial role in the advancement of Alzheimer’s research, especially in the early development stages of innovative studies like those conducted by Beth Stevens. Significant funding from agencies such as the National Institutes of Health has allowed researchers to explore fundamental questions about microglial function and its implications for neurodegenerative diseases. This financial support is essential, as it enables scientists to pursue long-term studies that can lead to transformative discoveries in understanding Alzheimer’s disease and developing effective treatments.
Moreover, public funding not only supports individual research endeavors but also fosters collaborative efforts across institutions. The Stevens Lab at Boston Children’s Hospital exemplifies how federal grants can facilitate partnerships among researchers, enhancing the multidisciplinary approach necessary for tackling complex health issues like Alzheimer’s. As the landscape of neurodegenerative research evolves, continued investment in foundational science will remain paramount, fueling innovation and translating basic research into practical solutions for millions affected by Alzheimer’s.
Importance of Curiosity-Driven Science in Neuroscience
Curiosity-driven science has always been at the forefront of groundbreaking discoveries in the field of neuroscience. The journey of researchers like Beth Stevens demonstrates the significance of following scientific curiosity, as it leads to unexpected findings that can impact health on a societal level. In her case, the exploration of microglial cells began with a simple interest in their role in the brain’s immune system but evolved into a revolutionary understanding of their involvement in neurodegenerative diseases such as Alzheimer’s. This highlights the essence of scientific inquiry—asking questions and exploring hypotheses can unveil new dimensions in our understanding of complex health issues.
Such an approach fosters an environment where innovative ideas can be cultivated, allowing researchers to think outside conventional frameworks. It encourages them to investigate the unknown, which is particularly essential in fields like neuroscience that carry vast complexities. As demonstrated by Stevens’ work, the commitment to curiosity-driven research can lead to pivotal insights that reshape our understanding and treatment of diseases. Encouraging this spirit within research institutions will ensure that the future of neuroscience continues to thrive, unveiling new pathways for combating neurological disorders like Alzheimer’s.
Potential Societal Effects of Alzheimer’s Research Advancements
Advancements in Alzheimer’s research not only hold the promise of improved individual health outcomes but also have significant implications for society as a whole. As the population ages, the burden of Alzheimer’s and other neurodegenerative diseases is expected to escalate. Innovations in understanding microglial function and developing effective treatments could alleviate this burden, potentially reducing the financial and emotional toll on families and healthcare systems. If successful therapies can be developed, they may significantly enhance the quality of life for millions, allowing individuals to maintain cognitive health longer and reducing the need for extensive care.
Additionally, increasing public awareness about Alzheimer’s research and the importance of funding can drive support for scientific endeavors. Efforts to translate basic research into applicable treatments can inspire public confidence in scientific progress, fostering a culture of support for continued funding and advocacy. As breakthroughs become publicized, they can rally community support, emphasizing the collective goal of improving health outcomes for those affected by Alzheimer’s. Ultimately, the societal impacts of advancements in Alzheimer’s research extend beyond just patients; they encompass families, caretakers, and the broader community, advocating for a healthier future.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s discovery?
Microglial cells are crucial to Alzheimer’s discovery as they act as the brain’s immune system, patrolling for signs of illness and clearing out damaged cells. Research, particularly from Beth Stevens’ lab, indicates that these cells’ aberrant pruning activities can contribute to neurodegenerative diseases like Alzheimer’s, shaping new pathways for treatment.
How has Beth Stevens contributed to Alzheimer’s discovery?
Beth Stevens has significantly advanced Alzheimer’s discovery by revealing how microglial cells affect synaptic pruning. Her research has highlighted the link between these immune cells and neurodegenerative diseases, paving the way for potential biomarkers and new treatments for Alzheimer’s disease.
What is the significance of microglial cells in neurodegenerative disease research?
Microglial cells play a vital role in neurodegenerative disease research, especially in Alzheimer’s disease. They are responsible for maintaining brain health but can contribute to disease progression when their pruning mechanisms malfunction, a key area of focus in Stevens’ groundbreaking studies.
How does Alzheimer’s treatment benefit from recent discoveries about microglial cells?
Recent discoveries about microglial cells benefit Alzheimer’s treatment by identifying how these immune cells contribute to disease progression. Understanding their function opens new avenues for targeted therapies, potentially improving outcomes for millions affected by Alzheimer’s.
What potential do new biomarkers hold for Alzheimer’s discovery?
New biomarkers hold significant potential for Alzheimer’s discovery as they can facilitate earlier detection of the disease. Research from Stevens’ lab emphasizes the role of microglial cells in identifying these biomarkers, which could lead to timely interventions in Alzheimer’s care.
What impact does the aging U.S. population have on Alzheimer’s discovery efforts?
The aging U.S. population is increasingly impacting Alzheimer’s discovery efforts, with cases expected to double by 2050. This demographic shift underscores the urgency of research, such as that conducted by Beth Stevens, which aims to develop new treatments and support for the growing number of Alzheimer’s patients.
What findings have been made regarding microglial cells and Alzheimer’s disease?
Research has shown that microglial cells can either protect or contribute to Alzheimer’s disease based on their pruning behavior. Aberrant pruning, as shown in studies by Beth Stevens, indicates that these immune cells can participate in the pathogenesis of neurodegenerative diseases, driving new therapeutic strategies.
How does curiosity-driven science impact Alzheimer’s discovery?
Curiosity-driven science, as seen in Beth Stevens’ work, is crucial to Alzheimer’s discovery. It encourages exploration beyond immediate disease implications, leading to foundational insights about microglial cells that ultimately enhance our understanding of Alzheimer’s and inform future treatments.
| Key Area | Description |
|---|---|
| Research Focus | Microglial cells and their role in the brain’s immune system. |
| Findings | Aberrant pruning by microglia can contribute to neurodegenerative diseases like Alzheimer’s. |
| Impact | Research can lead to new treatments and earlier detection of Alzheimer’s and other disorders. |
| Future Outlook | With an aging population, cases of Alzheimer’s are expected to double by 2050, increasing care costs significantly. |
| Funding Source | Majority of funding from National Institutes of Health and federal agencies. |
Summary
Alzheimer’s discovery has made significant strides thanks to the groundbreaking work of researchers like Beth Stevens. Her exploration of microglial cells has reshaped our understanding of neurodegenerative diseases, particularly how they affect synaptic health in the brain. As we face an increasing number of Alzheimer’s cases, Stevens’ research not only offers hope for future treatments but also emphasizes the importance of foundational science in driving medical advancements. Through continued support and funding, the pursuit of knowledge in this field may lead to life-changing treatments for millions.