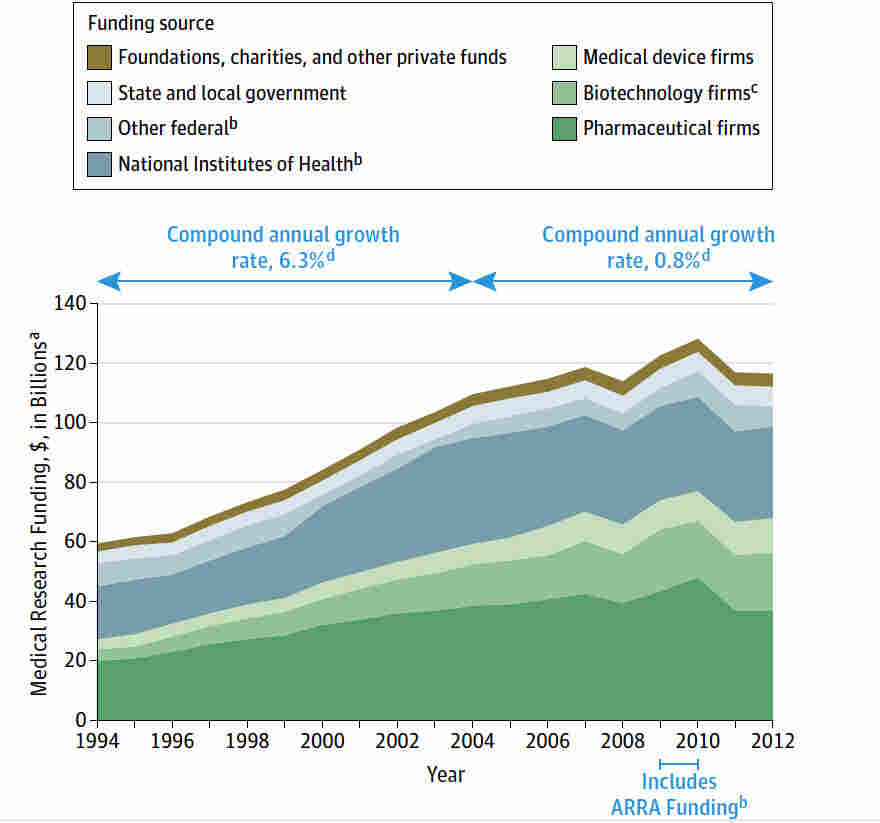
Medical research funding plays a pivotal role in advancing health care and ensuring the safety of patients involved in clinical trials. With robust funding mechanisms like NIH support, researchers can innovate, develop new treatments, and protect patient rights through rigorous oversight. However, the recent funding cuts have raised significant concerns over the impact on patient safety in research, as diminished resources can inhibit the crucial work of Institutional Review Boards (IRBs) tasked with ethical oversight. Without adequate funding, the integrity of research ethics oversight may falter, potentially putting participants at risk and fostering skepticism toward medical studies. This alarming situation underscores the urgent need to address the implications of funding limitations to safeguard both research progress and patient welfare.
Financial backing for healthcare studies, often referred to as medical research financing, is essential for fostering innovation and patient protection in clinical trials. This funding supports the vital activities of research oversight committees, ensuring that ethical guidelines are upheld and genuine consent is obtained from study participants. The current scenario highlights the setbacks caused by funding reductions, which can adversely affect patient experiences and compromise the thorough oversight provided by review boards. As we delve deeper into this topic, it becomes clear that maintaining adequate financial support is critical for the ongoing success of research initiatives and the trust of the communities they serve.
The Importance of Medical Research Funding
Medical research funding plays a critical role in advancing healthcare and ensuring the safety of patients involved in clinical trials. With adequate financial support, institutions can conduct thorough studies, utilize state-of-the-art technologies, and recruit highly qualified personnel who adhere to rigorous ethical standards. Recent funding cuts, such as the freeze on over $2 billion to Harvard, jeopardize these vital efforts. It hinders research institutions’ capabilities to innovate and improve treatment methodologies, ultimately affecting patient outcomes.
Furthermore, funding is essential not just for operational purposes but also for maintaining the infrastructure that ensures research integrity. This includes the Institutional Review Boards (IRBs) that are responsible for overseeing the ethical conduct of research. Without sufficient funding, the capabilities of IRBs to monitor compliance and safeguard patient safety are severely impaired, leading to potential ethical violations and risks to participants. Therefore, advocating for sustained medical research funding is crucial to protect both the research community and the patients they serve.
Patient Safety in Medical Research: The Role of IRBs
Institutional Review Boards (IRBs) play an indispensable role in safeguarding the rights and welfare of participants in medical research. They review research proposals to evaluate potential risks and ensure that informed consent processes are robust and transparent. The presence of a competent IRB system is essential for maintaining patient safety, as these boards act as ethical guardians, ensuring that the research adheres to all relevant laws and ethical guidelines. When funding cuts are imposed, the ability of IRBs to function effectively may be compromised, leading to lapses in oversight.
Moreover, IRBs foster trust between researchers and participants, which is crucial for successful clinical trials. Participants must feel confident that their welfare is being prioritized throughout the research process. As seen historically in events like the Tuskegee Syphilis Study, any erosion of trust can have devastating effects not only on current research efforts but on public engagement with medical studies in general. Thus, sustained funding for IRB activities is essential for mending past wounds and promoting future patient safety in research.
The Impact of Funding Cuts on Research Ethics Oversight
Funding cuts detrimentally impact the ethical oversight of medical research. The cancellation of grants and contracts diminishes the ability of IRBs and oversight bodies to perform their critical functions. These organizations rely on funding to not only maintain their operations but also to implement training programs, thorough reviews, and monitoring systems that ensure ethical compliance throughout the research process. With restricted resources, the potential for ethical breaches increases, putting participants at risk.
Moreover, when vital funding is lost, the research community may face growing skepticism from the public. Historical incidents of unethical research have already left an indelible mark on public trust in medical studies. If patients sense any reduction in ethical oversight due to budget constraints, they may become hesitant to participate in future studies. This cycle of mistrust could hinder advancements in medical research and innovation, leading to a decrease in the availability of new treatments and therapies.
NIH Funding Importance for Patient Safety
National Institutes of Health (NIH) funding is pivotal for facilitating research that guarantees the safety and rights of patients involved in clinical trials. NIH grants support comprehensive funding for ethically conducting research, including the essential processes managed by IRBs. These funds allow research institutions to recruit diverse patient populations, enabling inclusive studies that reflect the broader community. Without NIH funding, many crucial studies may not proceed, leaving gaps in knowledge that could benefit patient safety.
Additionally, NIH-funded projects often serve as precedents for best practices in research ethics. The requirements attached to these funds ensure that researchers adhere to stringent ethical standards, further emphasizing the importance of patient safety. The erosion of NIH funding will not only jeopardize current studies but could also deter future researchers from pursuing innovative ideas, thus stalling the progress necessary for improved healthcare solutions.
Challenges in Clinical Trials Without Sufficient Funding
Conducting clinical trials without sufficient funding leads to myriad challenges that can compromise patient safety and the integrity of research outcomes. Researchers may be forced to cut essential elements of study design, including adequate participant recruitment, compensation, and follow-up care. These compromises can result in fewer clinical sites participating in trials and diminished diversity among study participants, ultimately skewing the data and affecting generalizability to the broader population.
Furthermore, without the necessary funding, the continuous monitoring required to ensure participant safety may be overlooked. This disregard for rigorous oversight can lead to adverse events and put participants at risk. As the COVID-19 pandemic highlighted, swift and effective funding is essential for not just conducting trials but responding adequately to health crises. Therefore, ensuring robust funding for clinical trials is imperative for protecting patient safety and advancing public health.
The Historical Context of Patient Safety in Research
To understand the present significance of patient safety in research, it’s vital to examine the historical context that shapes current practices. Past atrocities, such as the Tuskegee syphilis experiment, revealed the necessity for strict ethical guidelines and oversight in research. These historical incidents reinforced the need for robust IRB protocols and ethical standards to prevent future abuses. By looking back, the research community can appreciate the progress made and recognize the ongoing challenges that funding cuts present to patient safety.
Moreover, these historical precedents emphasize the importance of ethical oversight in maintaining public trust. When patients entrust researchers with their health, there is an implicit understanding that their safety and welfare are prioritized. Sustainable funding is needed to uphold this trust by ensuring compliance with ethical standards and safeguarding participant rights. The lessons of the past continue to inform current practices and highlight the dire consequences that can arise when ethical oversight falters.
Community Impact of Reduced Research Funding
The community impact of reduced funding for medical research can be profound, especially regarding the safety and well-being of participants. Communities that actively engage in clinical trials typically do so with the expectation that their involvement will lead to advancements in medical knowledge and treatment options. However, when funding is cut, opportunities for communities to participate diminish, leading to a loss of trust in the research process and an overall reluctance to engage in clinical studies.
Moreover, the financial implications of reduced research funding extend beyond individual trials. Areas that depend on research grants to stimulate economic growth may face downturns, adversely affecting local economies. The ripple effect can lead to fewer healthcare advancements, which in turn can result in a stagnation of progress in treating diseases that disproportionately affect those communities. Thus, the importance of securing sufficient funding for medical research is not only about individual patient safety but also about the broader implication for community health.
Fostering Trust in Medical Research Through Funding Equity
Fostering trust in medical research is critical, especially in historically marginalized communities that may have experienced unethical research practices. Equitable funding for research initiatives can help bridge the gap by ensuring that diverse populations are represented and that their specific healthcare needs are addressed. This approach not only enhances the validity of research findings but also ensures that all patients feel their safety and interests are considered.
Moreover, promoting transparency in how research funds are allocated and utilized can help build trust between researchers and communities. Engaging with community leaders and stakeholders in the research process can foster a collaborative environment that emphasizes shared goals and mutual respect. By advocating for fair funding practices, we can create a more inclusive research landscape that prioritizes patient safety and reflects the diverse needs of society.
Strategies to Enhance Research Ethics Amidst Funding Challenges
To address the challenges posed by funding cuts on research ethics, institutions must adopt innovative strategies that prioritize patient safety and ethical oversight. Collaborating across institutions to share resources, data, and methodologies can help mitigate financial constraints while maintaining high standards for ethical compliance. For instance, creating consortiums where multiple IRBs collaborate on reviewing studies can streamline processes and reduce redundancy, ensuring efficient use of available funds.
Additionally, seeking alternative funding sources, such as non-profit grants and private sector partnerships, can help diversify funding streams and better support research efforts. Engaging with communities and stakeholders in determining research priorities can also foster ethical research practices that align with these groups’ values and needs. By proactively addressing funding challenges, the research community can enhance ethical oversight and continue to uphold the highest standards of patient safety.
Frequently Asked Questions
What is the impact of funding cuts on patient safety in medical research?
Funding cuts have a significant adverse impact on patient safety in medical research. When financial resources are restricted, it disrupts oversight systems essential for monitoring research protocols. This can lead to inadequate protection for participants, compromised ethical standards, and increased risks of harm. Moreover, halted studies and delayed research hinder the development of new treatments, ultimately affecting patient outcomes.
How does NIH funding importance relate to research ethics oversight?
The importance of NIH funding cannot be overstated, especially concerning research ethics oversight. NIH funds ensure that research involving human subjects undergoes rigorous ethical scrutiny through Institutional Review Boards (IRBs). These boards are crucial for safeguarding participant rights, ensuring informed consent, and minimizing risks. Without sufficient NIH funding, the IRB’s ability to maintain ethical standards and patient advocacy in trials is severely compromised.
What role do IRBs play in ensuring patient safety during clinical trials?
IRBs are fundamental to ensuring patient safety during clinical trials. They rigorously review study protocols, assess risks and benefits, and monitor ongoing research to protect participants’ welfare. Their oversight ensures that ethical standards are adhered to, informed consent is properly obtained, and that any potential adverse events are promptly addressed, creating a framework that prioritizes the safety of all research participants.
Why are funding cuts a concern for the future of patient safety in medical research?
Funding cuts pose a considerable threat to patient safety in medical research by limiting resources available for IRBs and other oversight mechanisms. This can lead to fewer safeguards being implemented, jeopardizing participant welfare and eroding public trust in clinical trials. As research becomes less well-funded, the quality and ethics of studies may decline, risking the integrity of patient safety protocols.
How does the system for patient safety in research depend on adequate funding?
The system for patient safety in research heavily relies on adequate funding to operate effectively. Financial support enables institutions to maintain IRB operations, training, and monitoring activities crucial for ethical research practices. Without sufficient funding, the capacity to implement comprehensive oversight diminishes, leading to potential risks for research participants and undermining confidence in the research process.
What strategies can be implemented to maintain patient safety amid funding cuts in medical research?
To maintain patient safety amidst funding cuts in medical research, several strategies can be employed. These include prioritizing funding for essential ethical oversight functions, fostering partnerships with private institutions to support IRB operations, enhancing community engagement to build public trust, and advocating for legislative measures that restore and protect research funding, ensuring that patient safety remains a priority even in challenging financial circumstances.
| Key Point | Details |
|---|---|
| Funding Freeze | The Trump administration froze over $2 billion in federal research grants to Harvard, disrupting oversight of patient safety in medical research. |
| Impact on Patients | The funding cuts jeopardize the rights and safety of participants in medical studies, with specific effects on the oversight roles of IRBs. |
| Role of IRBs | IRBs ensure the ethical conduct of research protecting participants through careful proposal review, risk assessment, and monitoring. |
| Historical Context | Historical abuses in medical research reinforced the need for rigorous oversight, leading to the establishment of IRBs. |
| Consequences of Cuts | Funding cuts have halted studies, restricted new site additions, and fostered distrust in the research community, complicating patient safety initiatives. |
| Future of Funding | Despite the halt, Harvard Medical School is providing support to continue essential research oversight. |
Summary
Medical research funding is critical for maintaining the safety and rights of research participants. The recent freeze on significant federal research grants has raised serious concerns about the ability to conduct ethical and safe medical studies. This disruption threatens the integrity of patient oversight by halting valuable research and undermining public trust in medical innovation. Ongoing support for collaborative research is essential to prevent public skepticism and maintain the health and safety of individuals participating in essential clinical trials.