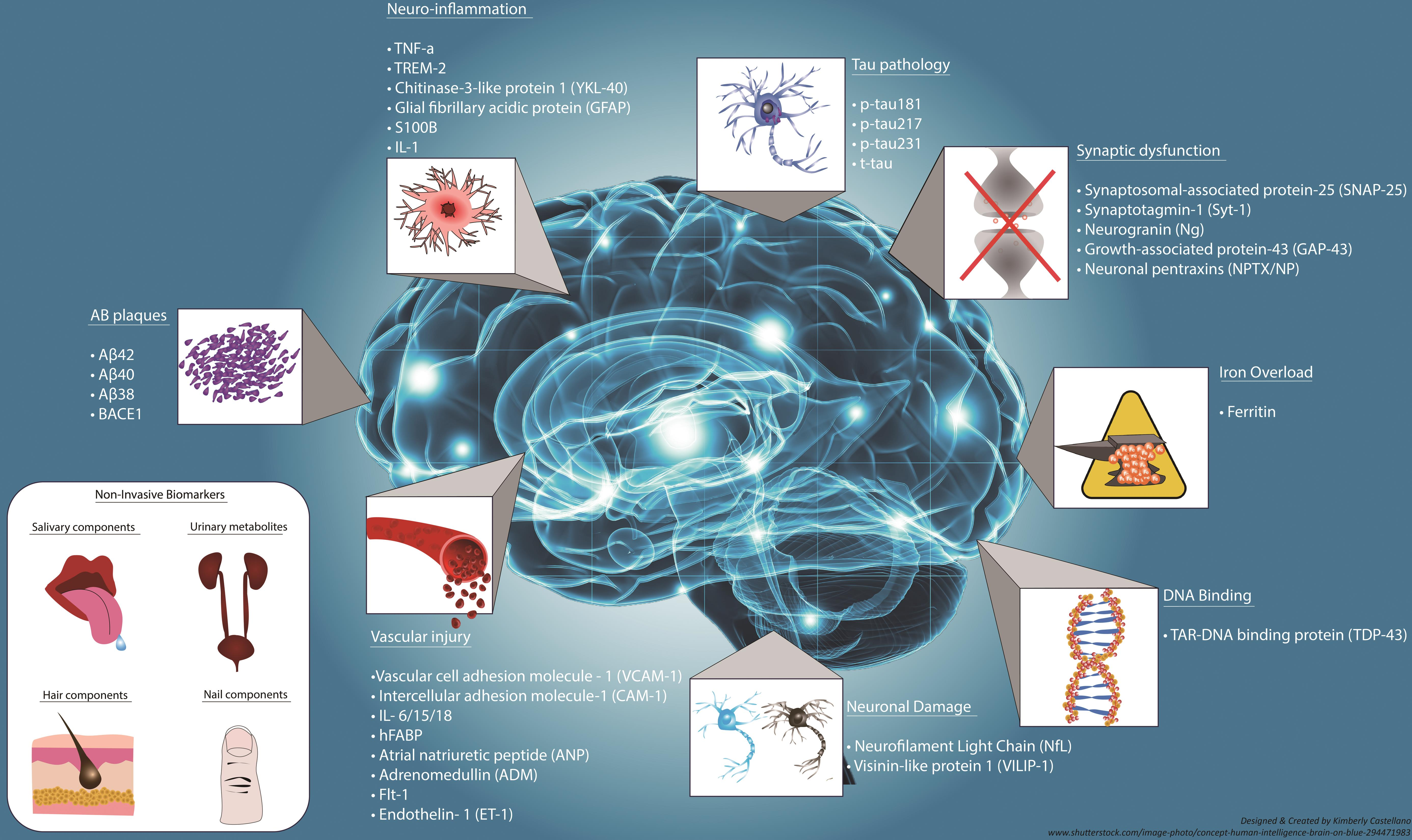
Alzheimer’s disease research has taken a transformative turn thanks to the groundbreaking work of scientists like Beth Stevens. Her studies on microglial cells—the brain’s immune system—reveal significant insights into how these cells manage synaptic pruning, a crucial process in neuronal communication. Abnormalities in this pruning can contribute to the progression of neurodegenerative diseases, including Alzheimer’s. As Stevens leads innovative explorations at Boston Children’s Hospital, her discoveries pave the way for new biomarkers and targeted treatments for millions affected by this incurable condition. This initiative represents not only a scientific breakthrough but also a ray of hope for the future of brain health and the fight against debilitating diseases.
Research on Alzheimer’s disease is reshaping our understanding of this debilitating cognitive decline. This field of study dives deep into the roles of brain immune responses and how factors such as synaptic pruning impact overall neuronal health. Investigators like Beth Stevens are uncovering the intricacies of microglial function, which is integral to the brain’s architecture and defense mechanisms. By examining the connections between these immune cells and neurodegenerative disorders, scientists are beginning to unlock new avenues for potential interventions. This innovative approach could significantly enhance diagnostic techniques and treatment options for those impacted by various forms of dementia.
The Role of Microglial Cells in Alzheimer’s Disease Research
Microglial cells are pivotal components of the brain’s immune system, acting as the first responders to cellular injury and pathology. In the context of Alzheimer’s disease, their function becomes particularly crucial as they engage in synaptic pruning—the process of eliminating unnecessary synaptic connections to maintain cognitive function. However, when microglial activity becomes dysregulated, it may lead to excessive pruning, contributing to cognitive decline and neurodegeneration. Researchers like Beth Stevens have been instrumental in revealing these relationships, highlighting how aberrant microglial responses can exacerbate conditions such as Alzheimer’s and Huntington’s disease.
Understanding the behavior of microglial cells is integral to developing targeted therapies for neurodegenerative diseases. Recent studies have shown that not only do these cells clear out damaged neurons, but they also play a significant role in remodeling neural circuits during pivotal brain development phases. This dual role underscores the complexity of microglial function, where improper activation can have deleterious effects on synaptic integrity. This line of inquiry into microglial cells is central to advancing Alzheimer’s disease research and paves the way for new therapeutic avenues that could fundamentally change patient outcomes.
Innovations in Neurodegenerative Disease Treatment
The innovative research led by scientists like Beth Stevens forms the backbone of recent advancements in understanding and treating neurodegenerative diseases, particularly Alzheimer’s. By studying microglial cells and their essential functions, researchers have begun to identify new biomarkers that can potentially signal the early onset of neurodegeneration. Detecting these markers early could lead to timely interventions and preventive strategies, ultimately enhancing the quality of life for individuals at risk of developing Alzheimer’s.
Moreover, the exploration of novel therapeutic agents aimed at modulating microglial activity represents an exciting frontier in Alzheimer’s disease treatment. By targeting the immune functions of these brain cells, scientists are looking to mitigate the harmful effects of excessive synaptic pruning while preserving the beneficial roles that microglia play in neuronal health. This approach not only emphasizes the importance of understanding the brain’s immune system but also showcases the potential for creating personalized medicine that caters to the unique neurobiological profiles of affected individuals.
The Importance of Basic and Curiosity-Driven Science
Basic and curiosity-driven science forms the foundation upon which groundbreaking discoveries in Alzheimer’s research are built. As highlighted by Beth Stevens, many significant advancements stem from seemingly unrelated scientific inquiries. For example, understanding the visual system of a mouse has led to insights about how microglial cells can shape and maintain neural circuits, demonstrating the interconnectedness of biological processes and the value of diverse research pathways. This approach not only fuels scientific innovation but also enriches the understanding of neurodegenerative diseases like Alzheimer’s.
Funding and support from agencies such as the National Institutes of Health are critical in nurturing basic research initiatives. Without the ability to explore fundamental scientific questions, researchers might lack the essential knowledge needed to address pressing health concerns like Alzheimer’s disease. This support enables scientists to delve into complex mechanisms like synaptic pruning and microglial cell function, fostering a culture of exploration that ultimately drives discoveries and enhances therapeutic strategies against neurodegenerative diseases.
Impact of Aberrant Synaptic Pruning
Aberrant synaptic pruning has been increasingly recognized as a critical factor in the progression of neurodegenerative diseases, particularly Alzheimer’s. When microglial cells become hyperactive, they may indiscriminately remove synapses, which leads to significant cognitive impairment. This process not only destabilizes neural networks but also accelerates neurodegeneration, making understanding the regulatory mechanisms involved vital for Alzheimer’s disease research. Insights into these mechanisms help identify potential therapeutic targets that can restore normal synaptic pruning.
Research on synaptic pruning emphasizes the delicate balance that microglial cells must maintain between promoting brain health and triggering neurodegeneration. By revealing the molecular pathways governing these processes, scientists can create targeted interventions aimed at modulating microglial activity and preventing excessive synaptic elimination. This knowledge challenges the traditional view of microglia solely as defenders of the brain, inviting a more nuanced understanding of their role in stabilizing cognitive functions and thwarting the advances of Alzheimer’s.
Synaptic Health and Cognitive Function
The health of synapses—critical connections between neurons—is vital for cognitive function, and their pruning by microglial cells must occur in a controlled manner. In Alzheimer’s disease, the balance of synaptic health is disrupted due to dysregulated microglial activity, which may lead to a loss of critical neural connections. Researchers are actively studying these dynamics, as understanding how to maintain synaptic health could lead to therapeutic strategies that preserve cognitive functions in affected individuals.
Emerging findings suggest that promoting healthy synaptic pruning, rather than inhibiting the microglial response altogether, may be key to developing effective treatments for Alzheimer’s. By re-establishing the protective mechanisms of microglial cells, it’s conceivable to enhance synaptic resilience and counteract cognitive decline. This approach indicates a paradigm shift in Alzheimer’s research that values the essential role of synaptic health in enduring cognitive function.
Beth Stevens: A Pioneer in Alzheimer’s Research
Beth Stevens has emerged as a leading figure in Alzheimer’s disease research, particularly due to her innovative studies on microglial cells and synaptic pruning. With a dedicated focus on the brain’s immune system, her work has illuminated the intricate relationships between microglia, synapses, and neurodegenerative diseases. By transforming conventional thinking about these immune cells, Stevens has opened new pathways for understanding and potentially treating Alzheimer’s disease and other conditions, encouraging a paradigm shift in neurological research.
Stevens’ profile illustrates the vital intersection of curiosity-driven research with practical health outcomes. As she notes, her initial explorations may have seemed distant from clinical implications, but they have paved the way for significant advancements in understanding neurodegenerative disease mechanisms. Her commitment to following scientific inquiry underscores the foundational importance of research in uncovering the complexities of diseases like Alzheimer’s and offers hope for the future of treatment and care.
Federal Funding’s Crucial Role in Neuroscience
The role of federal funding is crucial in driving neuroscience research, especially in fields aiming to address complex diseases such as Alzheimer’s. The foundational support from institutions like the National Institutes of Health allows researchers like Beth Stevens to pursue groundbreaking studies into microglial cells and neurodegenerative processes without the limitations often imposed by private funding. This financial backing fosters an environment where innovative ideas can flourish, leading to significant discoveries.
Continued investment in curiosity-led scientific research not only enhances our understanding of the brain’s immune responses but also seeks to develop new therapeutic strategies for Alzheimer’s disease. Federal support serves as a vehicle for advancing fundamental science, enabling researchers to explore the underlying mechanisms of neurodegeneration that could lead to breakthroughs in treatment. Such investments are essential for nurturing future advancements that may transform Alzheimer’s care and management.
The Future of Alzheimer’s Disease Research
The future of Alzheimer’s disease research is increasingly focused on understanding the multifaceted role of microglial cells and their implications for therapeutic interventions. As research progresses, scientists are encouraged to explore the nuanced interactions between these immune cells and neuronal health. The ongoing work by pioneers like Beth Stevens illustrates how discovering new biological pathways can lead to novel treatment approaches and targeted therapies that could ultimately reshape how Alzheimer’s is addressed.
Additionally, the integration of advanced technologies, such as single-cell RNA sequencing and genetic editing tools, is expected to further propel the field forward. These advancements will enhance researchers’ ability to dissect the complex cellular interactions that underlie neurodegeneration. By pursuing a better understanding of the brain’s immune system and the dynamics of synaptic health, the research community aims to develop innovative solutions for the millions affected by Alzheimer’s disease, emphasizing a bright future for investigative neuroscience.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells are integral to Alzheimer’s disease research as they function as the brain’s immune system, monitoring for illness and injury. They clear damaged cells and assist in synaptic pruning, a critical process that can become dysfunctional in neurodegenerative diseases like Alzheimer’s.
How does synaptic pruning relate to neurodegenerative diseases such as Alzheimer’s?
Synaptic pruning is essential for normal brain function, but in neurodegenerative diseases like Alzheimer’s, this process can become aberrant. Research led by scientists like Beth Stevens shows that improper pruning by microglial cells may contribute to the progression of Alzheimer’s disease, highlighting the need for targeted research in this area.
Who is Beth Stevens and what is her significance in Alzheimer’s disease research?
Beth Stevens is a prominent neuroscientist whose research at Boston Children’s Hospital and the Broad Institute focuses on the role of microglial cells in brain health. Her findings on how these cells engage in synaptic pruning have opened new avenues for understanding Alzheimer’s disease and developing potential treatments.
Are there any recent breakthroughs in Alzheimer’s disease research related to microglial cells?
Recent breakthroughs in Alzheimer’s disease research have demonstrated how microglial cells, by participating in synaptic pruning, can lead to both beneficial and detrimental effects. The Stevens Lab’s work has shown how faulty pruning mechanisms can exacerbate conditions like Alzheimer’s, paving the way for new therapeutic targets.
What impact does the dysfunction of the brain’s immune system have on Alzheimer’s disease?
The dysfunction of the brain’s immune system, primarily involving microglial cells, can lead to inadequate synaptic pruning and increased neurodegeneration. In Alzheimer’s disease, this dysfunction contributes to the accumulation of toxic proteins and the death of neurons, exacerbating the symptoms and progression of the disease.
How might new biomarkers from Alzheimer’s disease research improve diagnosis and treatment?
New biomarkers identified through Alzheimer’s disease research, particularly those linked to the activity of microglial cells and their role in synaptic pruning, could enhance early diagnosis and treatment strategies. By understanding how these immune cells contribute to neurodegeneration, researchers can develop targeted therapies that address the underlying mechanisms.
What is the significance of basic science in advancing Alzheimer’s disease research?
Basic science is crucial in advancing Alzheimer’s disease research as it provides foundational knowledge about brain function, including the roles of microglial cells in synaptic pruning. This fundamental understanding helps translate findings into practical therapies and interventions for Alzheimer’s and other neurodegenerative diseases.
| Key Point | Details |
|---|---|
| Microglial Cells | Act as the brain’s immune system, clearing dead cells and pruning synapses. |
| Research Contribution | Beth Stevens contributed to understanding how microglial cells can lead to neurodegenerative diseases like Alzheimer’s. |
| Impact of Aberrant Pruning | Improper pruning by microglial cells can result in Alzheimer’s and Huntington’s diseases. |
| Foundation for New Treatments | Research supports the development of biomarkers and medicines for Alzheimer’s disease. |
| Federal Support | Research has been primarily funded by the National Institutes of Health (NIH). |
Summary
Alzheimer’s disease research has made significant advancements, particularly through the work of neuroscientist Beth Stevens. Her exploration into microglial cells has reshaped our understanding of their role in brain health and disease progression. The realization that these immune cells can contribute to neurodegenerative conditions like Alzheimer’s has opened new avenues for treatment and care, highlighting the vital importance of basic science in combating this challenging disease.